The product liability attorneys at Zoll & Kranz have reason to believe the U.S. consumer market will be notified in the near future about a safety notice or recall from medical device manufacturer Stryker involving their LFIT CoCr V40 Femoral Heads, a component in a total hip replacement.
In previous litigations, we have seen safety warnings and recalls for a medical device begin in foreign countries before eventually being announced in the U.S. We have specifically seen this happen in another set of lawsuits regarding Stryker hip replacement components: Stryker Rejuvenate and AGB II.
That Stryker litigation is already in the settlement phase.
Recently, physicians in multiple foreign countries have been warned of potential safety issues associated with this device.
Some of these warning have come from governmental bodies, such as a Hazard Alert from the Australian Therapeutic Goods Administration and a recall in Canada reported by Health Canada. Other warnings have come from Stryker via a letter to orthopedic surgeons stating, “Stryker has received higher than expected complaints of taper lock failure for specific lots of certain sizes LFIR Anatomic CoCr V40™ Femoral Heads manufactured prior to 2011.”
The medical device attorneys at Zoll & Kranz believe U.S. consumers and patients deserve to know this information as soon as possible.
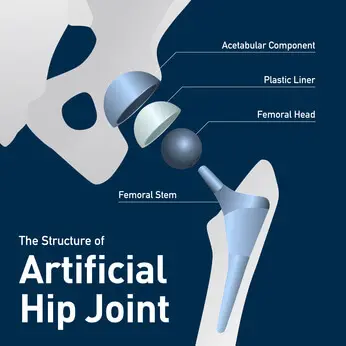
The femoral head of a hip replacement device is connected to the femoral neck by the taper lock.
Physicians abroad are being warned of taper lock failure with the Stryker LFIT CoCr V40 Femoral Heads.
Potential hazards associated with a taper lock failure may include:
These hazards may then result in harm to patients, such as:
The LFIT Anatomic CoCr V40 Femoral Heads are manufactured from a chromium/cobalt alloy.
As seen in other hip replacement lawsuits, excessive metallic debris may lead to metallosis and potentially require a revision.
The LFIT Anatomic CoCr V40 Femoral Head is designed to be used with Stryker modular hip devices such as:
If you are experiencing the adverse events above and have the Stryker LFIT CoCr V40 Femoral Heads hip implant, you may have a potential claim.
If you are unsure of which hip replacement device you have, our experienced mass torts product liability team can assist you in acquiring that information.
The Zoll & Kranz attorneys want to be sure patients are aware of the potential risk.
As always, we recommend you consult your physician if you have any medical concerns, especially about your Stryker hip replacement.
We focus our practice on helping patients harmed by medical devices and drugs, and we will bring our mass tort leadership experience to your case.
Our team is here to listen to your story and provide a free case evaluation and can be reached toll-free at (419) 827-3194 or by filling out the form here.
As we receive new information, we will update the website and blog but we welcome you to call us at the office for the latest news.

Michelle is a founding partner of Zoll & Kranz, located in Toledo, Ohio. Michelle has been a plaintiff’s lawyer for the entirety of her practice – over 32 years. She devotes the majority of her time to complex consolidated litigation and class action including advocating for people injured by medical devices, prescription medications, or corporate negligence.
This article has been written and reviewed for legal accuracy and clarity by the team of writers and attorneys at Zoll & Kranz, LLC and is as accurate as possible. This content should not be taken as legal advice from an attorney. If you would like to learn more about our owner and experienced Ohio injury lawyer, Michelle L. Kranz, you can do so here.
Zoll & Kranz, LLC does everything possible to make sure the information in this article is up to date and accurate. If you need specific legal advice about your case, contact us. This article should not be taken as advice from an attorney.
A serious injury can have life-altering results.
Don’t settle for less than you deserve, speak with an award-winning personal injury lawyer today.
For over 37 years, Zoll & Kranz has been fighting for clients who have been the victims of the wrongful death of a loved one.
Do you believe you’re entitled to compensation?
Use our Instant Case Evaluator to find out in as little as 60 seconds!
A serious injury can have life-altering results.
Don’t settle for less than you deserve, speak with an award-winning personal injury lawyer today.