-
Nursing home negligence encompasses a range of neglectful acts and abuse committed against residents of nursing homes, assisted living facilities, or other kinds of care facilities. It damages the mental, emotional, and physical health of
-
The Baxter Vascular Patch for peripheral vascular reconstruction is involved in a product recall. A FDA Class I recall is “a situation in which there is a reasonable probability that the use of or exposure
-
Reuters (9/27, Clarke), the AP (9/27) and the Wall Street Journal (9/27) reported on Friday that the Food and Drug Administration issued a warning that tigecycline (Tygacil), Pfizer Inc’s antibacterial drug, can increase the risk of death when
-
Today Reuters (2/14, Siebelt, Burger) reported that over 2,000 lawsuits in the US have been filed against the German pharmaceutical firm, Boehringer Ingelheim over its blood thinning medicine, Pradaxa (dabigatran etexilate). The claims allege that the
-
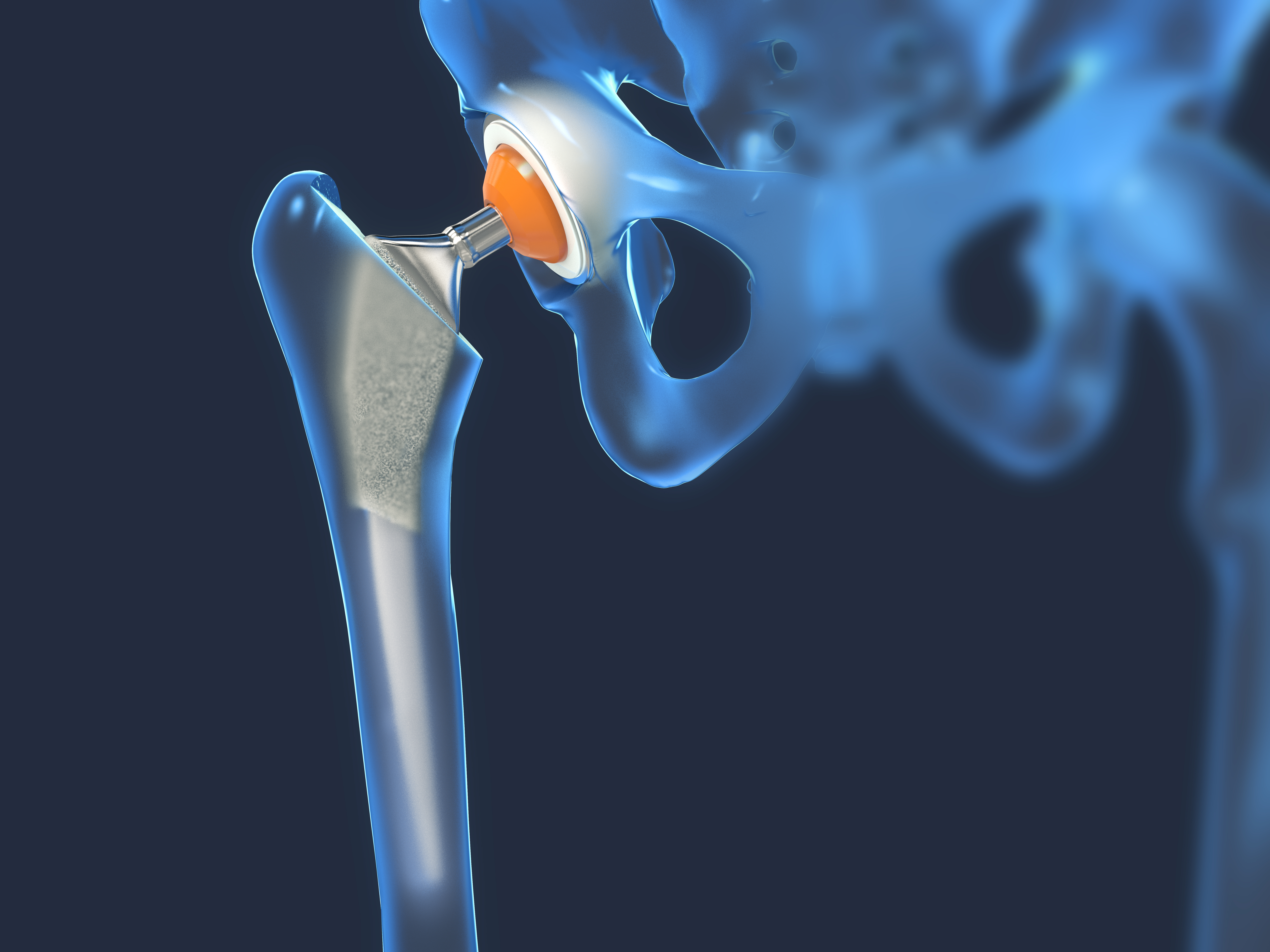
According to the Associated Press, more than two out of every 100 Americans now have an artificial joint, including knee and hip replacements. Not surprisingly, the number grows higher as people age. The AP reported that among people over
-
The law firm of Zoll & Kranz, LLC has filed lawsuits on behalf of four people the firm says were wrongly told they have Alzheimer’s disease. According to the Complaints filed in each case, each
-
According to the Wall Street Journal, Indian drug manufacturer Lupin Ltd. is recalling thousands of bottles of antibacterial drugs in the U.S. after finding that they failed to meet standards for impurities. The paper reported that the
-
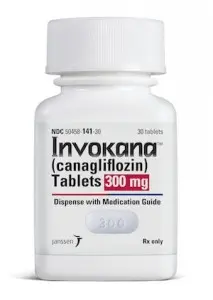
In March 2013, the FDA approved Invokana (canagliflozin) tablets for adults with Type 2 Diabetes to improve glycemic control. A New Class of Drugs When Invokana was approved, it was the first in a class
-
Have you suffered injuries due to Actos use? It should be noted that in 2011, the US Food and Drug Administration (FDA) announced that the use of Actos for more than one year could be
-
Have you experienced side effects like renal failure, increases in blood sugar and abdominal issues while taking the drug Acthar? You are not alone—according to multiple media reports, adverse events linked to the immune-system drug are
In the News
See Where Are Firm Has Been Featured in the News
At the law firm of Zoll & Kranz, LLC, our Multidistrict Litigation (MDL) and class action attorneys work hard to gain clients their deserved rights after a serious injury from dangerous drugs, defective medical devices or consumer products. Because of our nationally recognized work in these practice areas, news outlets from across the country frequently mention our firm and its involvement in personal injury cases.
Articles that Mention Our Law Firm, Clients and Cases
- The Courier: Report on ZK’s Force-Placed Insurance Lawsuit Against Wells Fargo
- ABC World News: Grieving Families Blame Heparin for Deaths
- ABC World News: What Went Wrong With Heparin?
- Bloomberg: Baxter’s Heparin Killed Mother and Son, Family Says
- Bloomberg: Baxter, Supplier Say Heparin Taint Was Deliberate
- Canadian Broadcasting Corporation (CBC): As families recall deaths, FDA investigates deliberate heparin contamination
- CNN: Heparin patients’ suffering recounted
- Medical News Today: Heparin Testimonies Before House Committee
- National Public Radio (NPR): Baxter CEO Says Heparin Purposely Tainted
You can also listen to this broadcast of NPR radio online.
- NBC24 WNWO: Toledo woman tells Congress of husband’s heparin death
- The National Law Journal: Suits roll in over recalled drugs; China may factor in heparin actions
- The New York Times: Heparin Contamination May Have Been Deliberate, F.D.A. Says
- Nightline from ABC News: Pharmaceutical Companies Must Take Responsibility
- The Toledo Blade: Paulding woman sets sights on justice after suffering ailments blamed on Bayer birth-control pill
- The Toledo Blade: $4.6 million settles airport-noise suit
- The Toledo Blade: 2 more lawsuits blaming heparin for 2 area deaths
- The Toledo Blade: 4 more suits filed blaming heparin for Toledo deaths
- The Toledo Blade: 5 sue diocese, claim priests abused them
- The Toledo Blade: 21 of 23 homeowners back plane noise-control program
- The Toledo Blade: Airport work gets OK to continue
- The Toledo Blade: All but 3 abuse cases are settled
- The Toledo Blade: Area family sues grower in spinach case
- The Toledo Blade: Area woman’s case joins surging flood of Vioxx complaints
- The Toledo Blade: Believers betrayed; Victims lived in silence for years until the truth began to be uncovered
- The Toledo Blade: Court: Port may be liable in nuisance suit; Air freight carrier ruled out as defendant
- The Toledo Blade: Deal calls for Resnick reprimand
- The Toledo Blade: Diocese hit by 6 sexual abuse suits; Legal papers allege pattern of cover-up
- The Toledo Blade: Drug, metal health boards near merger
- The Toledo Blade: Edison: Municipal power sales have limits
- The Toledo Blade: Heparin case awakens feelings of anger, fear
- The Toledo Blade: Heparin lawsuits transfer to Toledo
- The Toledo Blade: Hospitals hit over aid to uninsured
- The Toledo Blade: Judge upholds port authority’s airport project
- The Toledo Blade: Landowner gets reprieve from start of turnpike project
- The Toledo Blade: Legal chorus intensifies over noise from airport
- The Toledo Blade: Legal team to help in dispute
- The Toledo Blade: Liens filed on power plant; Area firms claim $5.5 million unpaid
- The Toledo Blade: Local family still seeks answers in loved ones’ deaths; drug company cited in lawsuit
- The Toledo Blade: Neighbors say the airport’s plan to manage noise isn’t enough
- The Toledo Blade: Noise dispute changes with flight path
- The Toledo Blade: Owner won’t let turnpike have land
- The Toledo Blade: Port accused of violating Ohio statute; Residents say air cargo hub didn’t get proper hearings
- The Toledo Blade: Port Authority ruled liable in lawsuit over noise
- The Toledo Blade: Port authority sues to hold insurers liable in noise lawsuits
- The Toledo Blade: Port authority zeroes in on image during retreat
- The Toledo Blade: Priest named in suit is placed on leave
- The Toledo Blade: Ruling has pension suit implications
- The Toledo Blade: Suit claims area priest abused boy in 1970s
- The Toledo Blade: Toledoans tell Congress of tainted-heparin grief
- The Toledo Blade: Pike foes open gate to Archbold exit
- The Toledo Blade: Victims lived in silence for years until the truth began to be uncovered
- The Toledo Blade: Widower sues drug company over heparin his wife used
- Thomson Reuters: Families tell U.S. lawmakers of heparin deaths
- U.S. House Commerce Committee on Energy and Commerce: The Heparin Disaster: Chinese Counterfeits and American Failures
- USA Today: Contaminated heparin deaths noted
- USA Today: Families of contaminated heparin victims tell stories of deaths
- WTOL News 11: Special Report: What killed my family?
- WTOL News 11: Toledo adds cases to mounting Heparin lawsuits
- Zimbio Entertainment: House Continues Investigation Into Heparin Contamination
In addition to the above news sources, WTVG-TV ABC 13, SACBEE, The Washington Post, Wisconsin State Journal as well as Yahoo News have also mentioned the work of our firm in articles that are no longer available to read online.
Talk With Experienced Class Action Lawyers Today
For more information about our involvement in these as well as other drug lawsuits and litigation, call Zoll & Kranz. Our firm offers individuals a complimentary case review by simply filling out an online form located on our site. Talk with us today to learn more about how our firm may be the right fit to represent your claim.