-
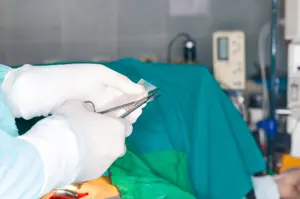
Medtronic is recalling its Duet External Drainage and Monitoring System due to faulty patient line tubing that may separate from the patient line connectors. The Duet EDMS externally drains and monitors cerebrospinal fluid and monitors
-
According to Reuters, earlier this month, a judge in West Virginia urged transvaginal mesh manufacturers to settle ongoing lawsuits with women who claim that the products have injured them, as cases have begun to pile up. The
-
Last fall, we wrote a post about how there were widespread reports of hidden ingredients in dietary supplements and nutritional products. Shockingly, according to the Journal of the American Medical Association (JAMA), in a study, more than
-
Tylenol (acetaminophen) has been linked to complications including liver failure and the drug is associated with an estimated 17,000 overdose situations each year. According to TIME, the rise in the sale of rise in prescriptions and non-prescription use of
-
Minimally invasive surgeries use a medical device called a Laparoscopic power morcellator (LPM). However, the Food and Drug Administration (FDA) discourages healthcare professionals from using this device for some procedures, because it could cause the spread of
-
According to Forbes, the US Food and Drug Administration (FDA) has issued a warning about using dietary supplements containing live bacteria or yeast with young children or people with compromised immune systems. The warning came after the
-
Several news outlets are reporting that drug maker Boehringer Ingelheim has been accused of withholding Pradaxa safety data from regulators, and doctors are beginning to use caution when prescribing the stroke-prevention medication. Pradaxa (dabigatran) has been
-
According to Bloomberg.com, the Danish maker of a vaginal mesh implant has agreed to pay about $16 million to settle lawsuits accusing the company of injuring women.Photo of mesh product Denmark-based Coloplast agreed in January
-
If you take Pradaxa (dabigatran), you should use caution. The drug has been linked to a higher risk for fatal health problems when compared to individuals using the blood thinner Warfarin. In a study of more
-
The Food and Drug Administration (FDA) has thoroughly reviewed the scientific literature on using transvaginal mesh to treat pelvic organ prolapse (POP) in women. Based on this review, the FDA says it has not seen enough evidence to support