In today’s blog, we continue our online discussion about Zofran’s risk of congenital birth defects by specifically addressing cleft palate and cleft lip injuries.
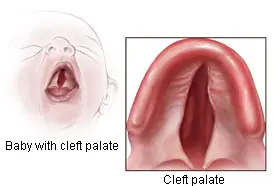
Cleft lip and cleft palate are birth defects that occur when a baby’s lip or mouth does not form properly during pregnancy. They are commonly referred to as “orofacial clefts.” During pregnancy, the right and left parts of the child’s lip and palate develop separately. The two parts should come together or “fuse.” A cleft lip or cleft palate occurs if this tissue does not join completely before birth.
The Centers for Disease Control and Prevention (CDC) has provided the public with facts about cleft palates and lips.
A cleft lip is an opening in the lip. As described by the CDC, the opening can be a small slit or a large opening that goes through the lip into the nose. It can be on one or both sides of the lip or, rarely, in the middle of the lip.
A cleft palate is an opening in the roof of the mouth. Babies may have both the front and back parts of the palate open, or they may have only one part open.
CDC: FACTS ABOUT CLEFT PALATE AND CLEFT LIP
The CDC has estimated that, each year in the United States, about 2,650 babies are born with a cleft palate and 4,440 babies are born with a cleft lip with or without a cleft palate. These children often have problems with feeding and talking. They also might have ear infections, hearing loss, and problems with their teeth.
Diagnosis is often made during pregnancy following an ultrasound. They are also diagnosed after birth, particularly a cleft palate. Certain types of cleft palates may not be diagnosed until later in life.
Often, surgery can close the lip and palate. Cleft lip surgery is often done before 12 months in age, and cleft palate surgery is done before 18 months. Additional surgeries may be required. Boston Children’s Hospital has posted a video (see below) on cleft palate and lips that is helpful in understanding the surgical repair options.
There are different potential causes of cleft palate and cleft lip, but it is not because the mother did anything wrong.
The nausea drug, Zofran, also known generically as Ondansetron, was developed by Glaxo Smith Kline (GSK) to treat nausea caused by chemotherapy. GSK, however, promoted Zofran “off label” (i.e. for an unapproved indication or for an unapproved group of patients) for morning sickness during pregnancy. However, unbeknownst to many mothers taking the drug and their physicians, Zofran crosses the placental barrier, and if taken during the first trimester can cause birth defects, including cleft palates and lips.
Researchers Anderka M, Mitchell AA, Louik C, et al. studied medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012;94: 22–30. That large control study by the Sloan epidemiology unit and the Centers of Disease Control and Prevention detected a 2-fold increased risk for cleft palate associated with ondansetron taken in the first trimester of pregnancy [odds ratio = 2.37 (95% CI, 1.28–4.76)].
Glaxo Smith Kline pled guilty to federal criminal charges in 2012. As a result, GSK paid the largest fine ever, $3 billion, for misconduct that included off label promotion of Zofran and other drugs, and paying kickbacks to doctors for prescribing Zofran off label. However, the plea did not resolve or settle cases for injuries to children born with these serious injuries. We believe children hurt by this drug are innocent victims and are entitled to compensation. This compensation is essential to help the families of these children shoulder the substantial cost of necessary medical treatment, including surgical repairs. As a result, we have agreed to represent multiple families in claims relating to Zofran.
If you would like more information about Zofran and its link with birth defects such as cleft palates and lips, or the claims being filed to help these children, please feel free to contact us toll-free at (888)841-9623. There is no fee for this call.

Michelle is a founding partner of Zoll & Kranz, located in Toledo, Ohio. Michelle has been a plaintiff’s lawyer for the entirety of her practice – over 32 years. She devotes the majority of her time to complex consolidated litigation and class action including advocating for people injured by medical devices, prescription medications, or corporate negligence.
This article has been written and reviewed for legal accuracy and clarity by the team of writers and attorneys at Zoll & Kranz, LLC and is as accurate as possible. This content should not be taken as legal advice from an attorney. If you would like to learn more about our owner and experienced Ohio injury lawyer, Michelle L. Kranz, you can do so here.
Zoll & Kranz, LLC does everything possible to make sure the information in this article is up to date and accurate. If you need specific legal advice about your case, contact us. This article should not be taken as advice from an attorney.
A serious injury can have life-altering results.
Don’t settle for less than you deserve, speak with an award-winning personal injury lawyer today.
FDA Warns Pfizer’s Drug Tygacil Raises Risk of Death
FDA Announces da Vinci Surgical System Robot Recall
FDA Issues Warning Over Phillips Heart Defibrillators
FDA Issues Warning About Medtronic Guidewire Recall
Unsterile Medical Device Recall: Zoll & Kranz, LLC is investigating the Seprafilm Class II Recall
For over 37 years, Zoll & Kranz has been fighting for clients who have been the victims of the wrongful death of a loved one.
Do you believe you’re entitled to compensation?
Use our Instant Case Evaluator to find out in as little as 60 seconds!
A serious injury can have life-altering results.
Don’t settle for less than you deserve, speak with an award-winning personal injury lawyer today.